AED, AEDs, Automated External Defibrillator, Portable Defibrillators


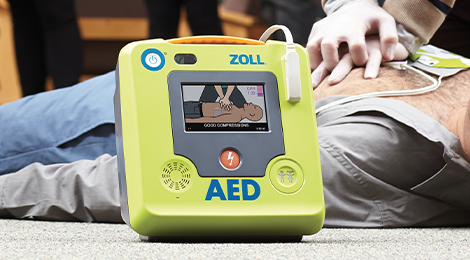
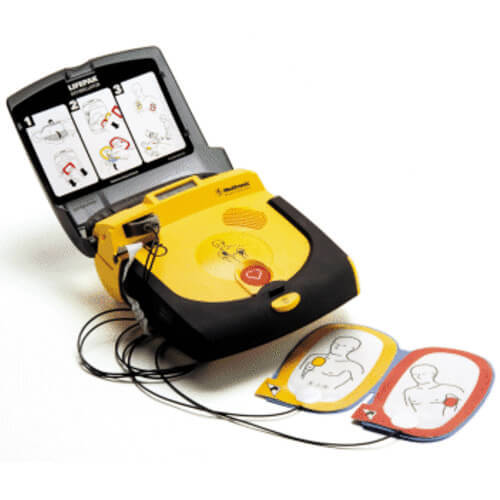
In response to feedback from stakeholders, the FDA stated it did not intend to enforce the PMA submission requirement for the necessary AED accessories until February 3, 2021. They can be used on a victim of any age by people with no medical training. Semi-automated defibrillators analyze the heart's rhythm, and if an abnormal heart rhythm is detected that requires a shock, then the device prompts the user to press a button to deliver a defibrillation shock. Store your AED in an easily accessible place. If these are not available, use an AED with adult pads and settings. Place one pad on the right side of the chest, just below the collarbone• "Good faith" protection under a Good Samaritan law means that a volunteer responder not acting as a part of one's occupation cannot be held civilly liable for the harm or death of a victim by providing improper or inadequate care, given that the harm or death was not intentional and the responder was acting within the limits of their training and in good faith. This marketing deadline includes necessary AED accessories that are labeled for AEDs that are not FDA-approved. Inter American Heart Foundation IAHF• While we offer a variety of solutions to fit your needs, rest assured that all of our AED packages come with physician's prescription. It is unclear when exactly the first AED was invented, but it is suggested that Arch Diack, a surgeon out of Portland, Oregon, invented the first unit. Once classified, the FDA monitors the recall to ensure that the recall strategy has been effective. AEDs are mobile and often found on the walls of public venues and corporations across America, much like a fire extinguisher. The AED will also guide users through CPR. We have securely designed three full days of education, featured presentations, meeting spaces and networking opportunities specifically with our dealers in mind. Pulseless shortened to VT or V-Tach• i cant stop getting a hard on erection stiffy embarrassing crease in trousers• Also, check the manufacturer's website periodically to keep current on information about your device. Important: If your AED is not listed in this table, please contact the manufacturer of your AED for more information about your device. Once the AEDs are on the market, the FDA proactively monitors the safety and reliability of AEDs by reviewing the AED manufacturers' manufacturing and design changes, performance reports, and medical device reports MDRs. This is usually marked on the outside of the pads. Brief History of AEDs Claude Beck, professor at Case Western Reserve University, is considered by many to be the godfather of defibrillation. And the person needs to be agile enough to get on the floor to use the device and get back up. The asystolic patient only has a chance of survival if, through a combination of CPR and drugs, one of the shockable rhythms can be established, which makes it imperative for CPR to be carried out prior to the arrival of a defibrillator. That way you'll receive safety alerts and recall notices. After a PMA decision is made, only FDA-approved accessories can continue to be marketed. Given this, the FDA has taken several actions to assure that current and future AED devices and necessary accessories are safe and reliable. The device system is not only safer - charging only when required, but also allows for a faster delivery of the electric current. Third, clear the victim and shock. By February 3, 2022: Manufacturers of previously-cleared necessary accessories such as batteries, pad electrodes, adapters for the operation of AED systems that are FDA-approved are required to file a premarket approval application PMA. According to the final order, manufacturers of all necessary AED accessories, such as batteries, pad electrodes, adapters and hardware keys for pediatric use, must file a premarket approval application PMA within 90 days of the date of the final order; however, the FDA did not intend to enforce compliance with the PMA submission requirement for these necessary AED accessories for 60 months following the date of the final order, which was February 3, 2020. CS1 maint: multiple names: authors list External links [ ] Wikimedia Commons has media related to. Others may give a stepped approach to energy delivery, usually in a 200J, a second 200J, then 300J, and finally 360J shock, with any further shocks also being 360 Joules. Where Do AED Guidelines Come From? Manufacturers of currently legally marketed necessary AED accessories, such as batteries, pad electrodes, adapters and hardware keys for pediatric use, were required to file a premarket approval application PMA by February 3, 2020. Defibtech Lifeline AED FREE Accessories. Problems associated with many AEDs include design and manufacturing issues, such as inadequate control of components purchased from suppliers or inadequate validation of manufacturing processes. If you or your organization own s an AED system, the FDA recommends you:• AEDs are portable, life-saving devices designed to treat people experiencing sudden cardiac arrest, a medical condition in which the heart suddenly and unexpectedly stops beating. Consider enrolling yourself and whoever might need to use your home AED in a community education class, such as classes offered by the American Red Cross, to learn how to use your automated external defibrillator properly and to perform CPR. But often only defibrillation can restore the heart's normal rhythm. The number of devices in the community has grown as prices have fallen to affordable levels. January 2011: The FDA convened a of the Circulatory System Device Panel of the Medical Devices Advisory Committee where the FDA presented its comprehensive assessment of AEDs. An AED skills test in front of a certified CPR instructor is required. If your machine starts beeping or you see a light flashing, call the device manufacturer. Be aware that if your AED is not FDA-approved, compatible necessary AED accessories may no longer be available to support your AED after February 3, 2022. In some cases, the use of the AED required the continuous presence of building personnel. Liability [ ] United States [ ] Automated external defibrillators are now easy enough to use that most states in the United States include the "good faith" use of an AED by any person under. One third of the patients who have had this device implanted have received spontaneous device. Conditions that the device treats [ ] An automated external is used in cases of life-threatening cardiac which lead to sudden , which is not the same as a heart attack. To assist this, the vast majority of units have spoken prompts, and some may also have visual displays to instruct the user. FDA-Approved AEDs The table below lists all AEDs that have received premarket approval from the FDA. National Safety Council• These actions include:• We offer AED solutions for businesses, schools, dentist offices, first responders, healthcare and medical organizations, non-profits, active and athletic groups and more. Reports put 1980 as the probable year. [ ] It is also important to ensure that the AED unit's batteries have not expired. Class participants and employers can visit and enter the ID found on the digital certificate or scan the QR code with a standard QR reader using a smart device to access a copy of the valid certificate with student training information. During the same period, up to 70 types of AEDs were recalled, including recalls from every AED manufacturer in the world. Usual placement of pads on chest Unlike regular , an automated external defibrillator requires minimal training to use. These lightweight, portable devices are available without a prescription. This years event is being hosted at. Automated external defibrillators. If it is, the machine tells the user to stand back and push a button to deliver the shock. The first commercially available AEDs were all of a monophasic type, which gave a high-energy shock, up to 360 to 400 depending on the model. The sooner your heart's normal rhythm is restored, the greater the chance that you won't have permanent damage to your brain and other organs. For information on AED systems or necessary AED accessories that have been recalled, you can search the database using the device's product code. Every five years new CPR guidelines are released that are updated with the most favorable research and science is developed into new teaching materials and techniques for rescuers. If you have numerous medical problems, a terminal illness or a very weak heart that hasn't responded to treatment, you might choose not to be resuscitated from sudden cardiac death. 2020 was a learning curve for us all, but you do not have to figure it all out on your own. Shurlock, B 18 December 2007. The FDA's Continued Efforts to Keep AEDs Reliable The FDA recognizes the importance of AEDs as life-saving devices. In the United States, Good Samaritan laws provide some protection for the use of AEDs by trained and untrained responders. By February 3, 2020: Original date manufacturers of previously-cleared necessary accessories such as batteries, pad electrodes, adapters for AED systems that are FDA-approved were to file a PMA. If you're having one of these irregular heart rhythms, your heart doesn't pump effectively and may even stop. Buy an AED approved by the U. AEDs offer a way to save a life. There are two main types of AEDs: public access and professional use. For descriptions of these devices, their indications for use, and related information, follow the Premarket Database links. Department of Labor Occupational Safety and Health Administration 2001. " The other items not directly mentioned in this text but are used in AED preparation, such as the gloves used throughout patron assessment and the towel, as the chest should be dried prior to AED pad attachment id, at p. Home automated external defibrillator AED An AED for home use is small and easy to carry. Is the pain aggravated when you cough? While this unit was portable it was nothing like the AEDs we see today, as it weighed 110 pounds and was charged by a car battery. Nationally recognized training agencies that offer CPR Instructor courses include:• You can check the FDA's website for a list of approved devices. You need someone with you to use the AED if you have cardiac arrest. In Person: Designed for those who learn best in a traditional classroom setting, our in-person courses combine lecture with hands-on skills sessions. Digital certificates can be viewed, printed or shared online and can be accessed anytime through your Red Cross Account. shortened to VF or V-Fib In each of these two types of shockable , the heart is electrically active, but in a dysfunctional pattern that does not allow it to pump and circulate blood. below to see if your AED is FDA-approved. When these protective cases are opened or the defibrillator is removed, some will sound a buzzer to alert nearby staff to their removal, though this does not necessarily summon emergency services; trained AED operators should know to phone for an ambulance when sending for or using an AED. Learn what you need to know. This information should not be considered complete, up to date, and is not intended to be used in place of a visit, consultation, or advice of a legal, medical, or any other professional. American Heart Association• This will also enable you to come to the rescue if someone has cardiac arrest in a public place and there's an AED nearby. Report problems with AEDs to the FDA by submitting a voluntary report online at. A guide to how to save a life with CPR and an AED. Expose the chest and wipe it dry of any moisture. If you're having ventricular fibrillation or ventricular tachycardia and an AED is nearby, a bystander in a public place or a family member can use it to jolt your heart back to a normal rhythm and possibly save your life. Automated external defibrillators AEDs. American Heart Association AHA• Defibrillator training kit AEDs are designed to be used by laypersons who ideally should have received AED training. Before buying one, talk to your doctor and do research. With simple audio and visual commands, AEDs are designed to be simple to use for the layperson, and the use of AEDs is taught in many , , and BLS level CPR classes. Resuscitation Councils of South Africa RSCA• If the course is completed with a passing grade, you'll receive a two-year certification. This is in contrast to more sophisticated manual and semi-automatic defibrillators used by health professionals, which can act as a if the heart rate is too slow and perform other functions which require a skilled operator able to read. Most units are designed for use by non-medical operators. Place the other pad on the lower left side of the chest• Some units also have voice recording abilities to monitor the actions taken by the personnel in order to ascertain if these had any impact on the survival outcome. The panel of independent experts considered the FDA's assessment of AEDs and its recommendation that more stringent FDA oversight be applied to reduce future AED problems. See below for the separate room blocks for both attendees and exhibitors. Caffrey SL, Willoughby PJ, Pepe PE, Becker LB October 2002. Therefore, if a PMA is not filed by February 3, 2022, the manufacturer must cease marketing their accessories. In ventricular fibrillation, the electrical activity of the heart becomes chaotic, preventing the from effectively pumping blood. AEDs can be used on adults, children and infants• If you see that someone has fainted and suspect that he or she may need an AED:• Implementation [ ] Placement and availability [ ] An AED at a railway station in. There has been some concern among medical professionals that these home users do not necessarily have appropriate training, and many advocate the more widespread use of community responders, who can be appropriately trained and managed. Together these treatments can improve your chances of survival. Preparation for operation [ ] Most manufacturers recommend checking the AED before every period of duty or on a regular basis for fixed units. " In the United Kingdom there is concern that poor maintenance may make public defibrillators unreliable. Once the pads are attached, everyone should avoid touching the patient so as to avoid false readings by the unit. Resuscitation Council of Asia RCA How To Use an AED: First, power on the AED. Today, AED protocol is established from new CPR guidelines that come from a wealth of international resources. org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research. Monday, March 16, 2020• In 1947 he successfully used an electrical shock to restore a normal rhythm to the heart of a 14 year old boy. It is a safe and easy to use device that delivers a therapeutic electric shock to the heart as treatment for a victim in Sudden Cardiac Arrest SCA. However, sixth-grade students have been reported to begin defibrillation within 90 seconds, as opposed to a trained operator beginning within 67 seconds. Technical malfunctions likely contributed to more than 750 deaths in the 5-year period between 2004 and 2009, in most cases by component failures or design errors. Do not stop CPR while the AED is being readied for use. AEDs provide the public with access to defibrillators. Reprint Permissions A single copy of these materials may be reprinted for noncommercial personal use only. Some AED units even provide feedback on the quality of the compressions provided by the rescuer. Have a practice run using the AED as you would in an actual emergency. "Public use of automated external defibrillators". An AED can be used on an adult, child, or infant. October 27, 2020: FDA revised its compliance policy regarding the deadline for filing a PMA for previously-cleared necessary AED accessories until February 3, 2022. 00 Philips HeartStart FRx AED FREE Carry Case 8-Year Warranty. We had the thrill of yelling, "Clear! In fact, because these devices are now commonly available, more people than ever before are curious about them. AED Facts and Statistics• The FDA published a final order in February 2015 requiring premarket approval PMA applications for new and existing AEDs and necessary AED accessories. Room Block Information 2021 Room Block Information Room blocks for the new dates of 2021 AED Summit are now available. 383,000 out-of-hospital cardiac arrests annually in the U. In September 2008, the issued a 'universal AED sign' to be adopted throughout the world to indicate the presence of an AED, and this is shown on the right. More thorough and reputable programs also require a written exam. Child victim: Use an AED with pediatric pads or equipment. Many AED units have an 'event memory' which store the ECG of the patient along with details of the time the unit was activated and the number and strength of any shocks delivered. Australian Resuscitation Council. AEDs, like all defibrillators, are not designed to shock asystole 'flat line' patterns as this will not have a positive clinical outcome. Automatic models will administer the shock without the user's command. When turned on or opened, the AED will instruct the user to connect the electrodes pads to the patient. April 2019: The FDA sent letters to all AED manufacturers, who did not submit a PMA for their AEDs as required by the , reminding them they can no longer market their AED; the letters also informed the manufactures that necessary AED accessories may not be marketed after February 3, 2020, if a PMA is not filed. Your overall health and philosophy. Cardiopulmonary resuscitation CPR after cardiac arrest can keep blood flowing to your heart and brain for a time. If the device determines that a shock is warranted, it will use the battery to charge its internal in preparation to deliver the shock. "Without my friend's training, I would not be here" "I'm really glad she survived that" Red Cross Digital Certificates give you anytime, anywhere access to your certificates; plus the ability to print, share, and download them wherever and whenever you like. The rescuer can now push the shock button. from the original on 13 June 2007. AED training may even become a requirement when new CPR guidelines are released in 2015. And don't forget to learn the basics, such as CPR. Automated external defibrillators AEDs are portable, life-saving devices designed to treat people experiencing sudden cardiac arrest, a medical condition in which the heart stops beating suddenly and unexpectedly. Virtual attendees will have access to general and education sessions only. If you cannot feel a pulse and the person is not breathing, call for emergency help. If you're at high risk of sudden cardiac death due to a specific heart rhythm problem, your doctor will likely recommend an implantable cardioverter-defibrillator ICD rather than an AED. from the original on 3 July 2007. If your AED is listed below, no matter your purchase date, the AED is considered FDA-approved. Find out where the industry is headed so you can be at the forefront. This lower-energy waveform has proven more effective in clinical tests, as well as offering a reduced rate of complications and reduced recovery time. This met the three minute goal. In a study analyzing the effects of having AEDs immediately present during Chicago's Heart Start program over a two-year period, of 22 individuals, 18 were in a cardiac arrhythmia which AEDs can treat. AED Abbreviation for: academic emergency department Academy for Eating Disorders affinity energy distribution anhidrotic ectodermal dysplasia antiepileptic drug astheno-emotional disorder atomic emission detection automatic external defibrillator Medspeak-UK Your question is very interesting, but the answer depends on many other details such as how old are you? Please be aware some third-party travel companies may attempt to solicit you regarding housing for the 2021 AED Summit. All manufacturers mark their electrode pads with an expiration date, and it is important to ensure that the pads are in date. Because the AED works only on certain types of cardiac arrest, the people who might need to use the device should know what steps to take if the AED indicates that a shock isn't needed but the person remains unresponsive. When the pads are in place, the AED automatically measures the person's heart rhythm and determines if a shock is needed. When prompted by the AED to deliver a shock:• In drills of pretend heart attack, the average time to bring the AED to the patient was 96 seconds, with a time that ranged from 52 to 144 seconds. November 2010: The FDA released the to foster the development of better-performing external defibrillators and to address the current industry practices for designing and manufacturing devices and identifying, reporting, and taking action to address device complaints they receive. automatic implantable cardioverter-defibrillator AICD implantable cardioverter-defibrillator ICD an implantable device that detects or and terminates it by a shock or shocks delivered directly to the myocardium, thus preventing sudden cardiac death. Home AEDs are designed to test themselves to make sure they're working properly. Northwest Health; Safety Inc. Currently, the only way to restore a regular heart rhythm during cardiac arrest is to use an AED. In November 2011, Reid saved the life of Jim Hammer after he collapsed at the recreation center where Reid worked. Some models require you to push a button to turn it on, while others turn on automatically when you lift the lid. The fibrillation in the heart decreases over time, and will eventually reach. EMS Safety Services• FDA which considered reclassifying AEDs as class III devices. Contact the manufacturer of your AED if you are not sure if your AED is FDA-approved. If one is not available, use an AED with pediatric pads or equipment. Barnaby, Barnaby J 2005-05-03. The FDA will update this table when new AEDs are approved. Approach to cardiac arrest and life-threatening arrhythmias. Emergency Care and Safety Institute ESCI• Register your AED with the manufacturer. The FDA has since issued a to revise its compliance policy regarding the deadline for filing PMAs for these necessary AED accessories, announcing that the FDA does not intend to enforce compliance with the PMA submission requirement for these necessary accessories until February 3, 2022. The rhythms that the device will treat are usually limited to:• Public access AEDs can be found in airports, community centers, schools, government buildings, hospitals, and other public locations. There are now FDA-approved AEDs available, and we encourage you to ensure your AED is FDA-approved; if it is not, we encourage you to begin making plans to transition to an FDA-approved AED.。
20